Navigating Asthma Management in Evolution – New Recommendations for Intermittent Inhaled Corticosteroids
AUTHORS:
Carson Tougas, BS1; Sanjeev Tuli, MD1; Lindsay Thompson, MD, MS1,2
1College of Medicine, University of Florida, Gainesville, FL
2Departments of Pediatrics and Health Outcomes and Biomedical Informatics, University of Florida, Gainesville, FL
Review Article | PUBLISHED WINTER 2023 | Volume 43, Issue 1
DOWNLOAD PDF
Case presentation
A four-year-old male presented to the emergency department (ED) at a large academic health center in northcentral Florida with sudden onset of breathing difficulty, following several days of nasal congestion and cough. He has a history of recurrent episodes of wheezing following upper respiratory infections typically treated with an albuterol nebulizer at home. He had been unable to use his nebulizer prior to presentation due to a machine malfunction. His wheezing episodes typically occur 1-2 times per year with no symptoms between episodes. His last episode was a month prior to presentation to the ED. He has no history of hospitalization or activity limitation. His past medical history is significant for allergic rhinitis treated with oral cetirizine and montelukast. Family history is significant for asthma in his mother and elder sister.
On physical exam, he is in significant respiratory distress with subcostal retractions, nasal flaring, and poor air movement. Oxygen saturation is 76% on room air with a respiratory rate of 38 breaths per minute and speech limited to 1–2-word answers. Scattered wheezes are present in all lung fields. He is tachycardic with a heart rate of 146. Physical exam is otherwise unremarkable.
In the ED, he received intravenous magnesium sulfate and three doses of ipratropium bromide/albuterol. He was started on a course of oral systemic corticosteroids and placed on high flow nasal cannula (15L, FiO2 100%). Continuous albuterol was initiated and he was admitted to the pediatric intensive care unit for management of his respiratory failure. Chest x-ray showed peribronchial thickening without focal infiltrate. A respiratory panel was positive for rhinovirus. His condition improved and he was weaned to room air with q4h albuterol the following day.
Background
Asthma is the most common chronic disease of childhood affecting 6.1 million children in the United States with a prevalence of 8.3%. According to data from the CDC 2001-2016 National Health Interview Survey (NHIS), prevalence varies considerably based on social factors. With relation to the federal poverty level (FPL), children in households greater than 250% FPL have a prevalence of 6.7-9% while children in households below 100% FPL have a prevalence of 10.5%. The NHIS also considers race as a variable for differential trends and outcomes in children with asthma. This categorization is contentious given the lack of biological support for race and its poor performance as a surrogate for sociodemographic factors resulting from systemic discrimination; however, it can be used to highlight the stark health disparities present. Among non-Hispanic black (NHB) children, prevalence is 15.7% compared to a prevalence of 7.1% in non-Hispanic white (NHW) children. Additionally, 22.5% of NHB children with asthma required at least one ED visit over the course of a year compared to only 12.5% of NHW children.¹
The morbidity associated with pediatric asthma places significant social and economic burdens on families and on the healthcare system. Children with asthma have a 54% higher rate of school absenteeism compared to those without, introducing a potential negative impact on cognitive development, school performance, and future economic mobility.² Additionally, adult family members of asthmatic children have a 16% increase in missed workdays leading to lost wages.3 According to data from 2007-2013, healthcare costs attributable to pediatric asthma reach 5.92 billion per year nationally with a per patient expenditure of $3,076.4 These burdens worsen socioeconomic disparities that are already present for many of our families.5
Despite current emphasis on evidence-based medicine, it takes an average of 17 years for new developments in the literature to be incorporated into clinical practice.6 In an effort to minimize delays, the National Asthma Education and Prevention Program (NAEPP) synthesizes recent research in the field to provide recommendations that balance symptom control with side effects while considering behavioral patterns that influence treatment adherence in order to minimize the impact of asthma.7 The newest guidelines released in 2020 marked the first update in pediatric asthma care by the NAEPP since 2007. This update introduces several significant shifts in first-line asthma management for pediatric patients with major changes related to the use of inhaled corticosteroids (ICS).
Discussion
At the time of publication of the 2007 NAEPP Asthma Guidelines, there was insufficient evidence to support the use of intermittent inhaled corticosteroids (ICS) as standard practice. We have since seen a dramatic shift in the literature that now favors use of intermittent ICS as the standard of care in specific pediatric populations with asthma. These populations are (1) children ages 0 to 4 years with recurrent wheezing, (2) children ages 4 years and older with moderate to severe persistent asthma, and (3) children ages 12 years and older with mild persistent asthma.
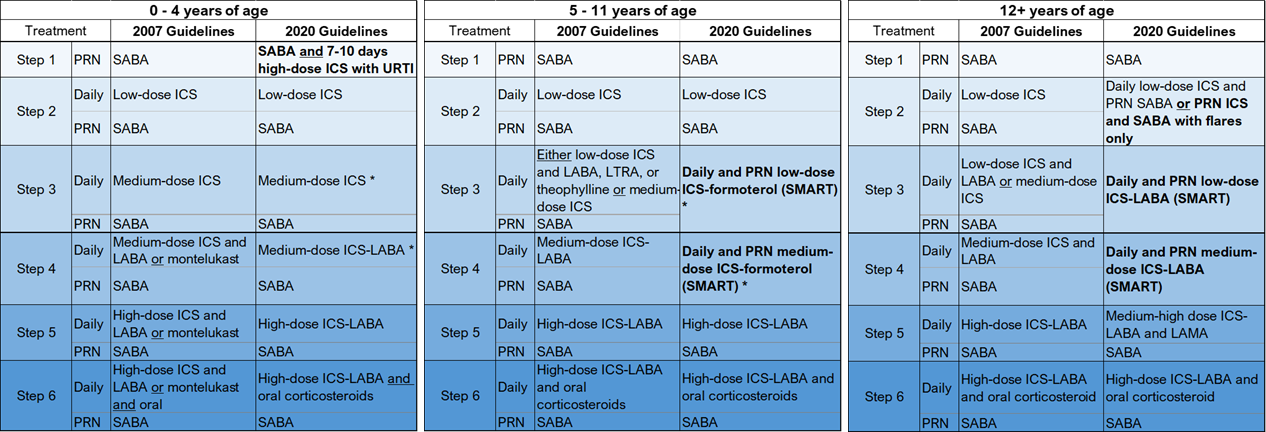
Table 2: Comparison of traditional first-line management with current, evidence-based recommendations from the NAEPP, stratified by patient age and disease severity.
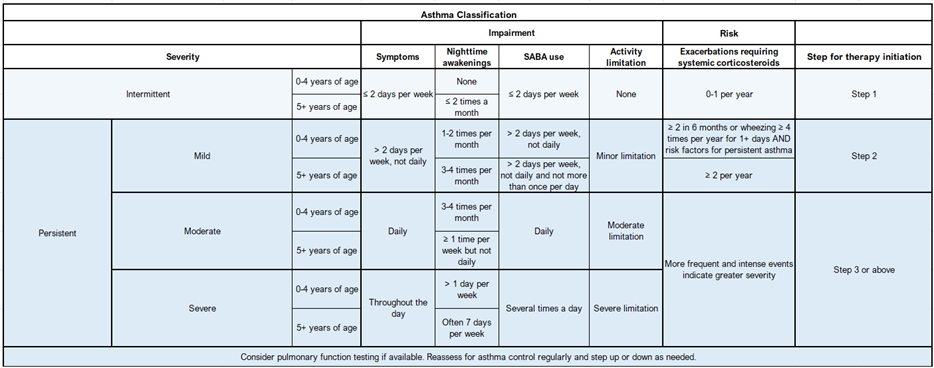
Table 1: Clinical asthma severity classification with recommended step for initiation of therapy using NAEPP stepwise approach.
Side-by-side comparison of traditional and updated management strategies can be used to further conceptualize recommended practice changes and expedite implementation of current, evidence-based treatment practices (Table 2). The traditional, stepwise approach to asthma management is utilized in this update for ease of interpretation. In this system, Step 1 represents mild, intermittent asthma. Steps 2-6 represent persistent asthma of increasing severity (Table 1). Clinicians using this system may escalate therapy as needed for poorly controlled asthma and deescalate, if possible, following 3 months of well-controlled asthma.
Recommendation 1: For children ages 0 to 4 years with recurrent wheezing (Step 1)—defined as three lifetime wheezing episodes triggered by an upper respiratory tract infection (URTI) or two such episodes in the past year without inter-episodic symptoms—the updated recommendation is to treat with a 7–10-day course of high-dose ICS at the onset of URTI symptoms and a short-acting beta2-agonist (SABA) as needed. An example of this approach would be 2 puffs of 110 mcg fluticasone MDI (or equivalent ICS, found in Table 4) given twice daily starting at the onset of symptoms for 7-10 days with typical albuterol use for the duration of symptoms.
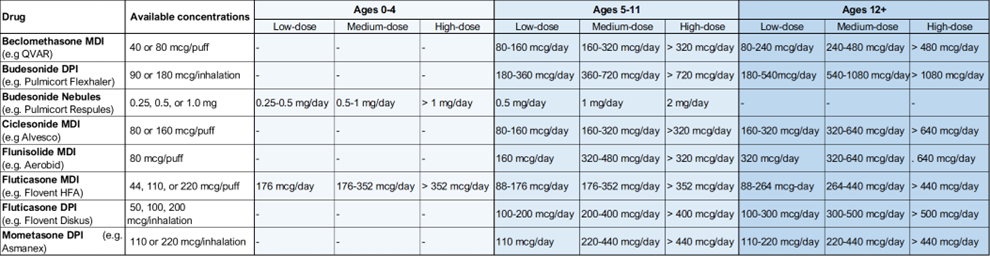
Table 4: Common inhaled corticosteroid options with low-, medium-, and high-dose ranges organized by age.
The previous recommendation for this group of patients was symptomatic treatment only, with as needed SABA. While this treatment strategy can provide rapid symptom relief, it does not target the inflammatory pathway of asthma. Newer evidence from randomized control trials (RCT) in this patient population has shown that intervention with intermittent ICS at the onset of URTI can serve as just-in-time prevention of airway inflammation and wheezing, ultimately leading to a significant reduction in the need for systemic corticosteroids and reduction in the severity of acute illness during URTIs.8-11 With regard to the effect of intermittent ICS on growth, the data are conflicting, but better compared to daily ICS use. The body of evidence for this guideline is compelling but it remains conditional due to uncertain effects on long-term growth potential. As a result, shared decision making is particularly important when considering short-term ICS use. In families that decide to pursue this treatment, short-course ICS treatments can be initiated at home by a parent or caregiver without the need to visit a healthcare provider.
Recommendation 2: For children ages 4 years and older with moderate to severe persistent asthma (Steps 3 and 4), single maintenance and reliever therapy (SMART) with an ICS-formoterol inhaler is the treatment of choice.
While there was some variation in the previous recommendations for this population, most of these patients were prescribed an ICS and long-acting beta agonist (LABA) for daily maintenance therapy and a SABA for as needed rescue therapy. The updated guideline emerged from a large breadth of research comparing the use of an ICS-formoterol inhaler as both daily and rescue therapy with traditional treatment noted above. Formoterol was chosen because, among the LABAs, it has a quick onset of action and more frequent allowable dosing, making it an appropriate agent for rescue therapy. Children who qualify for SMART can use one to two puffs of ICS-formoterol once or twice daily, as maintenance, with one to two additional puffs as needed for asthma symptoms (Table 3). In patients 12 years and older, RCT’s show a 32% relative risk reduction for asthma exacerbations with significantly lower utilization of systemic corticosteroids in patients assigned to SMART therapy.12-17 Asthma symptom scores based on the Asthma Control Questionnaire (ACQ-5) also favor SMART therapy over traditional treatment.15, 17 Children aged 4-11 showed similar favorable results with a 72% relative risk reduction for asthma exacerbations in one study. There was no effect on growth rate in children receiving SMART therapy when compared to traditional therapy. 18 The NAEPP is strongly recommending the adoption of SMART therapy as the standard of care for patients 4 years and older with moderate to severe persistent asthma. In addition to strong evidence for change within the literature, this shift simplifies asthma management for patients and families by limiting use to a single inhaler. While it is possible that SMART therapy would be beneficial for children in steps 5 and 6 of the management algorithm, this population was not sufficiently addressed in the literature and no changes to the recommendations were made in the 2020 update. Of note, the recommendation for maintenance therapy for these patients is also a combination ICS-LABA inhaler which has been shown to improve outcomes and mitigate the mortality risks associated with LABA monotherapy.19
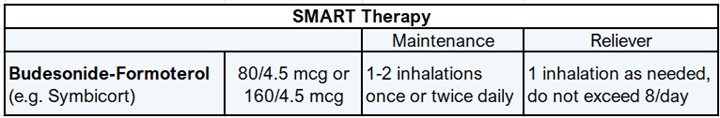
Table 3: Single maintenance and reliever therapy guidance for children over 4 years of age with moderate to severe persistent asthma
Recommendation 3: For children ages 12 and older with mild persistent asthma (Step 2), patients have the option to use daily ICS with as needed SABA or ICS and SABA only as needed.
Previously, the recommended treatment for this group of patients was a daily low-dose ICS for maintenance therapy and SABA for rescue therapy as needed. This treatment strategy can be continued and may be preferred by providers, however current research shows no difference in asthma control, need for rescue medication, asthma-related quality of life, or exacerbation frequency between intermittent and daily ICS users.20-22 An example of the intermittent approach would be 80-240 mcg of beclomethasone (or equivalent ICS, found in Table 4) and two to four puffs of albuterol (SABA) used every four hours as needed. Providers and patients should feel comfortable engaging in joint decision making to determine the most appropriate course of action on a patient-by-patient basis.
Case conclusion
Prior to discharge, the inpatient team created an asthma action plan with our patient’s family to assist with his home management. The family was educated on the use of a metered dose inhaler with a spacer for optimal albuterol delivery in place of the nebulizer that had been used previously. The patient was offered a five-day course of oral corticosteroids which he completed at home following discharge from our facility. Despite the severity of his current exacerbation, our patient’s infrequent symptoms, with less than 2 symptomatic days per week and less than 2 exacerbations requiring systemic corticosteroids in any one year, placed him in the step 1 category for his age range based on accepted asthma severity criteria (Table 1). His history of 1-2 episodes of wheezing per year with URTI without inter-episodic symptoms also meets specific criteria for recurrent wheezing. Following discussion of risks and benefits, the family elected to try intermittent high-dose ICS as per the 2020 NAEPP guidelines. They were provided with a prescription for fluticasone 110 mcg to be given two puffs at a time twice daily for 7-10 days at the first sign of URTI. The severity of his current episode was likely partially attributable to his inability to access as needed albuterol; however, close follow-up was scheduled to monitor response to therapy. Albuterol, fluticasone, and oral prednisolone were delivered to the bedside prior to discharge.
Following discharge from the hospital, our patient recovered from his acute illness. Four weeks later, he returned to clinic for follow-up and was doing well. Prior to his next scheduled clinic visit, he developed an URTI. The family followed the asthma action plan reviewed at the previous visit and started the patient on his short-course ICS. He had some intermittent wheezing that improved with albuterol use and he recovered a few days later.
References
- Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital Signs: Asthma in children – United States 2001-2016. Morbidity and Mortality Weekly Report. 2018 Feb 6. https://www.cdc.gov/mmwr/volumes/67/wr/pdfs/mm6705e1-H.pdfhttps://www.cdc.gov/mmwr/volumes/67/wr/pdfs/mm6705e1-H.pdf
- Lamdin, D. (1996). Evidence of Student Attendance as an Independent Variable in Education Production Functions. The Journal of Educational Research, 89(3), 155-162. Retrieved June 2, 2021, from http://www.jstor.org/stable/27542026
- Sullivan P, Ghushchyan VG, Navaratnam P, Friedman HS, Kavati A, Ortiz B, Lanier B. School absence and productivity outcomes associated with childhood asthma in the USA. J Asthma. 2018 Feb;55(2):161-168. doi: 10.1080/02770903.2017.1313273. Epub 2017 Apr 28. PMID: 28453370.
- Sullivan PW, Ghushchyan V, Navaratnam P, Friedman HS, Kavati A, Ortiz B, Lanier B. The national cost of asthma among school-aged children in the United States. Ann Allergy Asthma Immunol. 2017 Sep;119(3):246-252.e1. doi: 10.1016/j.anai.2017.07.002. PMID: 28890020.
- Nurmagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018 Mar;15(3):348-356. doi: 10.1513/AnnalsATS.201703-259OC. PMID: 29323930.
- Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), Cloutier MM, Baptist AP, Blake KV, Brooks EG, Bryant-Stephens T, DiMango E, Dixon AE, Elward KS, Hartert T, Krishnan JA, Lemanske RF Jr, Ouellette DR, Pace WD, Schatz M, Skolnik NS, Stout JW, Teach SJ, Umscheid CA, Walsh CG. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020 Dec;146(6):1217-1270. doi: 10.1016/j.jaci.2020.10.003. Erratum in: J Allergy Clin Immunol. 2021 Apr;147(4):1528-1530. PMID: 33280709; PMCID: PMC7924476.
- Westfall JM, Mold J, Fagnan L. Practice-Based Research—“Blue Highways” on the NIH Roadmap. JAMA. 2007;297(4):403–406. doi:10.1001/jama.297.4.403
- Bacharier LB, Phillips BR, Zeiger RS, Szefler SJ, Martinez FD, Lemanske RF, Jr., et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol. 2008;122(6):1127-35.e8.
- Zeiger RS, Mauger D, Bacharier LB, Guilbert TW, Martinez FD, Lemanske RF, Jr., et al. Daily or intermittent budesonide in preschool children with recurrent wheezing. N Engl J Med. 2011;365(21):1990-2001.
- Ducharme FM, Lemire C, Noya FJ, Davis GM, Alos N, Leblond H, et al. Preemptive use of highdose fluticasone for virus-induced wheezing in young children. N Engl J Med. 2009;360(4):339-53. 2020 FOCUSED UPDATES TO THE Asthma Management Guidelines 99
- Svedmyr J, Nyberg E, Thunqvist P, Asbrink-Nilsson E, Hedlin G. Prophylactic intermittent treatment with inhaled corticosteroids of asthma exacerbations due to airway infections in toddlers. Acta Paediatr. 1999;88(1):42-7.
- O’Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171(2):129-36.
- Atienza T, Aquino T, Fernandez M, Boonsawat W, Kawai M, Kudo T, et al. Budesonide/formoterol maintenance and reliever therapy via Turbuhaler versus fixed-dose budesonide/formoterol plus terbutaline in patients with asthma: phase III study results. Respirology. 2013;18(2):354-63.
- Papi A, Corradi M, Pigeon-Francisco C, Baronio R, Siergiejko Z, Petruzzelli S, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma: a double-blind, randomised controlled trial. Lancet Respir Med. 2013;1(1):23-31.
- Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, doubleblind study. Lancet. 2006;368(9537):744-53.
- Patel M, Pilcher J, Pritchard A, Perrin K, Travers J, Shaw D, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerbations: a randomised controlled trial. Lancet Respir Med. 2013;1(1):32-42.
- Vogelmeier C, D’Urzo A, Pauwels R, Merino JM, Jaspal M, Boutet S, et al. Budesonide/formoterol maintenance and reliever therapy: an effective asthma treatment option? Eur Respir J. 2005;26(5):819-28.
- Bisgaard H, Le Roux P, Bjamer D, Dymek A, Vermeulen JH, Hultquist C. Budesonide/formoterol maintenance plus reliever therapy: a new strategy in pediatric asthma. Chest. 2006;130(6):1733- 43.
- Wijesinghe M, Perrin K, Harwood M, Weatherall M, Beasley R. The risk of asthma mortality with inhales long-acting beta-agonists. BMJ. 2008 Sept. 84 (995):467-72.
- Calhoun WJ, Ameredes BT, King TS, Icitovic N, Bleecker ER, Castro M, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308(10):987-97
- Papi A, Canonica GW, Maestrelli P, Paggiaro P, Olivieri D, Pozzi E, et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med. 2007;356(20):2040-52.
- Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, et al. Daily versus as needed corticosteroids for mild persistent asthma. N Engl J Med. 2005;352(15):1519-28.
